In today’s world, lithium batteries are everywhere. The most common places they can be found are in household items, such as remote controls, watches, smart phones, and laptops. However, larger batteries are used in medical equipment, fire apparatuses, construction equipment, and other industrial equipment.
While lithium batteries have opened up many new opportunities in both our homes and work lives, they also carry certain risks—especially during transportation when factors like pressure, temperature, and damage can come into play.
In order to successfully (and legally) transport batteries, there are a minimum of set requirements. We will ensure you understand the vocabulary, their history, why they are regulated, and the dangers these types of batteries can impose.
Where did lithium batteries come from?
Batteries have a much longer history and development than most people might think. As a matter of fact, it’s believed the first crude battery was created over 2,000 years ago!
In 1938, scientists discovered what is now called the “Baghdad Battery.” Found in Khujut Rabu, Baghdad, the battery is thought to be from roughly 250 BCE. It consisted of an earthen jar with an opening that was sealed with an asphalt plug. The plug held in place a copper sheet rolled into a tube which was capped at the bottom with a copper disc held in place by more asphalt. A thin rod was stuck through the upper asphalt plug and hung down into the center of the copper tube which wasn’t touching any part of it. When the jug was filled with vinegar or fermented grape juice, a small volt was created.
Basically, the first battery was a jug made of dirt with metal rods that was powered by either vinegar or wine. Can you imagine your phone today being powered by THAT?
The term “battery” can be traced back to 1749 when Benjamin Franklin coined the term while referring to a set of linked capacitors he used for his experiments with electricity. While these were not true batteries, they did manage to store a charge and the term “battery” became the common term.
The first acknowledged true battery was the “Voltic Pile” which was invented in 1800 by Italian Scientist Alessandro Volta. From Volta’s battery came a series of other common battery types that are still used today, including Nickel-Iron, Lead Acid, and, of course, Lithium. And, although the first lithium battery experiments began in 1912, the first lithium battery wasn’t commercially available until 1991.
Why are lithium batteries so highly regulated?
As lithium batteries increase in capacity and strength, so does their danger. Since January 2019, the Federal Aviation Administration (FAA) has recorded over 50 incidents of lithium battery fires on aircraft alone.
One of the more tragic examples of the dangers of transporting lithium batteries occurred on September 3, 2010. A UPS Boeing 747 flying from Dubai to Cologne developed an in-flight fire, causing the plane to crash, killing both pilots onboard. This was the first fatal air crash recorded that was caused by lithium batteries—but it wouldn’t be the last.
As more lithium battery incidents occurred, and more data developed, a few things became clear as to WHY lithium battery fires are such a concern. Unlike a typical fire, lithium battery fires are a form of chemical fire—this means the normal modes of putting out the fire are ineffective. Lithium batteries do not respond well to typical (or even chemical) fire extinguishers or water, and they don’t need oxygen to burn so suppression systems are not as effective. Specialized chemicals are required to put out a lithium fire, and if those chemicals aren’t available, the only effective way to put out the fire is to let it burn.
In addition, lithium battery fires burn HOT. Your average house fire burns at 593ºC (or 1100ºF). To put it into perspective, the highest recorded lithium battery fire burned at 750ºC (or 1382ºF). That’s a big difference! What’s even more important to note though is that aluminum (which is a big part of airplane construction) melts at 660ºC (or 1221ºF). See the problem?
We have included a link that will take you to a video which shows how a pallet of lithium batteries (like a pallet of cell phones) in a plane’s cargo hold would react should there be a fire. This particular video shows a pallet of 5,000 Li-Ion batteries.
Why are lithium batteries so dangerous?
Lithium batteries are small, lightweight, powerful, and long-lasting. Although they are very safe overall, accidents and incidents do happen, and when they do, they may be extremely dangerous, resulting in an explosion or fire.
This is what the inside of a lithium battery looks like:
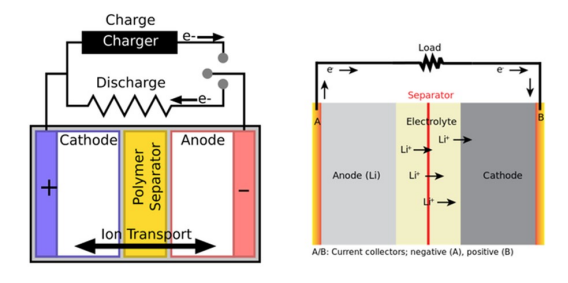
Lithium batteries are made to deliver big results, but weigh virtually next to nothing. In order to achieve this, you need lightweight components, including thin partitions between the cells and a thin outer covering. Unfortunately, this means the partitions and coating are pretty fragile, making it so they can be easily punctured, crushed, damaged, or worn out. If the battery becomes damaged, a short circuit occurs, potentially creating a spark and igniting the lithium (which, as we mentioned before, if really flammable).
Another possibility is the battery can heat to the point of thermal runaway, which means there is an excess of heat where the temperature continues to rise. The increased heat of the contents then puts pressure on the battery, producing an explosion.
There are some ways to minimize the risk of a fire or explosion:
- Avoid storing batteries in hot places (i.e., in a hot car, under your pillow, in your pocket);
- Don’t store batteries all together, as each of them gives off heat; and
- Don’t overcharge batteries (NOTE: Using any charger besides the one intended for the battery increases the risk of damage).